From MyLife Technologies
LUMC, the Leiden University Medical Center in the Netherlands, today announced the approval and enrolment for theMILESTONE study. Using ceramic skin patches, this phase 2A clinical trial will evaluate the safety, immunogenicity, and attractiveness in applying a 5x lower quantity of MODERNA mRNA vaccine against COVID-19 as an easy-to-use skin patch, compared against normal muscle jabs. The ceramic skin patches and preparations are provided by MyLife Technologies BV, a start-up company located next door in the Leiden Bio Science Park. The outcome of the trial is expected by the end of 2022.
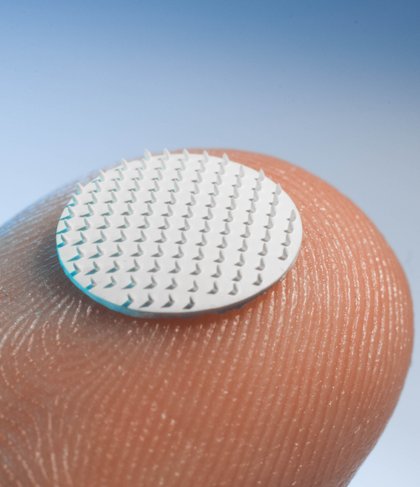
•Simple vaccine patch with much lower vaccine doses than injection and no needle anxiety
• Easier to store vaccines, fewer side effects and lower costs
• Contributes significantly to early containment of potential epidemics/pandemics in low- and middle-income countries
• Lowers threshold for HPV vaccination
• Collaborationwith international vaccine companies and global NGOs
MyLife Technologies, which focuses on the development of ceramic vaccine patches for the simple, efficient and painless administration of vaccines through the skin, has raised €3.5 million from Fare Capital BV, SAL Beheer BV, informal investors and the existing shareholders. With this capital, MyLife in Leiden will start the production of the vaccine patches for clinical studies and invest with partners in demonstration projects against COVID-19, HPV and in vaccine therapy against certain types of cancer. MyLife foresees a next series A investment round of around €15 million in 202 for the construction of a pilot plant and expansion of activities to the US and Asia.
Click here for further details.

December 2020: MyLife Technologies is awarded the MIT R&D Development grant with partner 20Med from Enschede (NL). MyLife combines its intradermal microneedle arrays with 20Med's mRNA nanoparticles as a rapid-response technology for new outbreaks of infectious diseases. The proposal was ranked first place out of 20+ high tech propositions in the East Region of the Netherlands. The projectmay also be relevant for cancer therapies using mRNA- or DNA-vaccines as nano particles.
September 2020: MyLife is awarded the prestigious Eureka InnoWide grant with its market screening project to define development and entry of improved HPV-vaccines for Indian markets together with Indian Partner Transform SciTech. The proposal was ranked first place out of 700+ enteries.
July 2020: MyLife closes €0.9 Mln as first half of its intermediateround to establish the company as aTechnology Platform provider for vaccine delivery. The company focuses on vaccine delivery in the skin to enhance the performance of modern vaccines, both for therapeutic and for prophylactic uses.
The proceeds are used to: 1) scale-up its micro-needle processing technology; and 2) demonstrate with partners vaccine delivery projects using its proprietary ceramic nano-porous Micro Needle Array technology.

June 2020: Outcome of the First-in Human studies with healthy volunteers concludes the CeraMAPs(TM), with and without a commercially available peptide drug loading to be safe and applicable for intradermal drug delivery as conducted by Center of Human Drug Research in July-August 2019
March 2020: Mylife Technologies is selected to enter the EIT Health 'Start-ups meetPharma' project. The project combines companies with Pharma partners looking for specific technologies or opportunities.

January 2020: MyLife Technology appoints Mike de Leeuw as new CEO, with focus on the transition to offering its CeraMAPs/npMNA Technology Platform to especially vaccine developers in applications for cancer therapies and infectious diseases, coming from the previous single product development strategy).

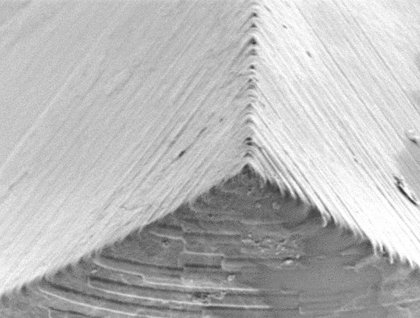
October 2019: Second US patent granted to MyLife Technologies for its ceramic nanoporous MicroNeedle Array (npMNA) technology.
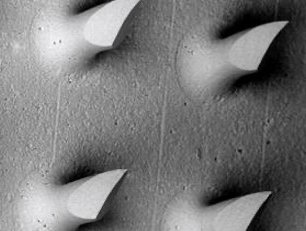
October 2019: EIT Health, Headstart grant awarded to MyLife Technologies for its NextGen SkinPatch project.
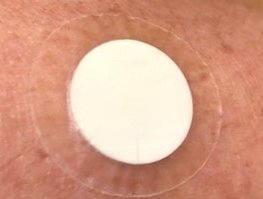
PRESSRELEASE
Leiden, Netherlands, June 28, 2019
First volunteer dosed with microneedle patch of MyLife Technologies at CHDR
MyLife Technologies started a first-in-human study with its ceramic nanoporous MicroNeedle Arrays (npMNA) technology at Centre for Human Drug Research (CHDR) in Leiden (NL) today. npMNA patches loaded with a pharmaceutical peptide formulation are being tested in healthy volunteers.
In many cases pharmaceutical peptides are parenterally administered. Existing alternatives for peptide delivery are among others intranasal sprays or subcutaneous implants. npMNA patch administration could lead to reduced peak concentrations and modified drug release and should overcome several of the drawbacks of the existing delivery routes: npMNA patches are minimally invasive, pain-free and allow for self-administration. The drug formulation is stored in the nanopores of the npMNA devices and diffuses into the patient once the npMNA patches have been applied onto the skin. Drug release rates from the nanopores can be tuned. This makes MyLife Technologies’ npMNA patches a versatile drug delivery system.
“We have entered an exciting new phase in the development of our npMNA technology”, says Pieter Jan Vos, CEO of MyLife Technologies, “We have found a valuable partner in CHDR, an experienced clinical CRO in the field of novel concepts and dermatology”.
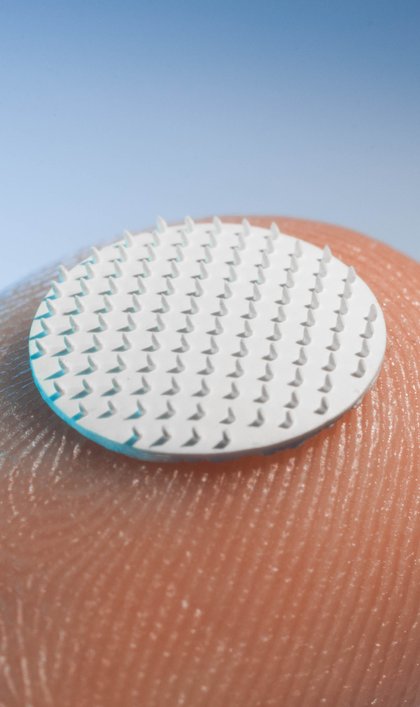
PRESSRELEASE
Porous microneedles for pain free and patient friendly medication
Leiden, November 29th 2018 – To further develop their technology – a patch holding porous microneedles – MyLife Technologies received a €300.000 investment from investment fund UNIIQ. The patch provides an important alternative for current drug administration via microneedle or oral routes (tablets, capsules, etc.). The patch provides the opportunity to deliver drugs and vaccines transdermally, which makes it a pain free route of administration. Additionally, the patch overcomes a number of limitations that current pharmaceutical patches are faced with. The investment was announced by Henri Lenferink, mayor of the city of Leiden, during the Dutch Life Sciences conference.
See also this doc.

Microneedles 2018, 7 June 2018
We are proud to present our npMNA technology for intradermal vaccination during Microneedles 2018 in Vancouver! The results of an in vivo vaccination study with Diphtheria- and Tetanus Toxoid performed together with University of Utrecht (NL) were presented in a poster session. This study was done with financial support from ADITEC Europe.
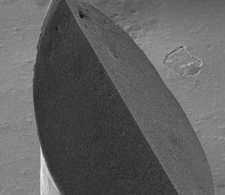
MyLife Technologies secures new growth capital,
14 November 2016
A consortium of informal investors has invested 0.5 million € in MyLife Technologies, a med tech company developing dermal delivery solutions based on its platform technology of ceramic nano-porous MicroNeedle Arrays (npMNAs). MyLife Technologies will use this investment to accelerate the development of pharmaceutical applications of its npMNA technology.
The company focuses on applications in the field of prophylactic vaccines, therapeutic vaccines and specific small molecules. The npMNAs are made of biocompatible ceramic material and are combined with a dermal patch, allowing easy self-application by the patient. The nanopores of the MNA devices are used for storage of pharmaceutical compounds. This minimally invasive application method is pain-free and is an effective way to overcome the stratum corneum, the skin’s main barrier.
MyLife Technologies is a spin-off from University of Twente, MESA+, Institute for Nanotechnology, The Netherlands. In June 2015, the company opened its own lab in the facilities of BioPartner Center at Leiden Bio Science Park. Technology patents have been obtained in Europe, USA, Japan and China.
CEO Pieter Jan Vos is very pleased: “This new capital is an important milestone for MyLife Technologies. It allows us to expand our lab facilities and our team. We will intensify and expand our development pipeline”.

C.J. Kok Award for best dissertation of the year 2015
Koen van der Maaden PhD, Development Officer at MyLife Technologies, has won the C.J. Kok Award for best dissertation of the year 2015! Koen van der Maaden’s research, described in his thesis “Microneedle-mediated vaccine delivery” on which he recently obtained his PhD degree cum laude, has brought painless vaccination using microneedles major steps forward. From the jury report: “The jury of the C.J. Kok prize was impressed by the excellence of the scientific work, the broad array of techniques applied by Koen and the major societal impact of the research. Microneedles are not only painless, they will also allow more easy storage of vaccines in dry form and thus eliminating the requirement for refrigeration and the applicator designed will upon further development allow administration by non-medical personnel. Koen’s discoveries thus have brought future application of worldwide painfree vaccination against diseases-like the measles, polio and other infectious diseases important steps closer.”
See also this link

In 'Radio EenVandaag'; an interview with Pieter Jan Vos (Spoken Dutch)
Listen to the radio segment here.
Marijke Roskam of BNR news radio interviews Pieter Jan Vos (Spoken Dutch):
Listen to the radio segment here.

Copyright © 2023
All rights reserved
